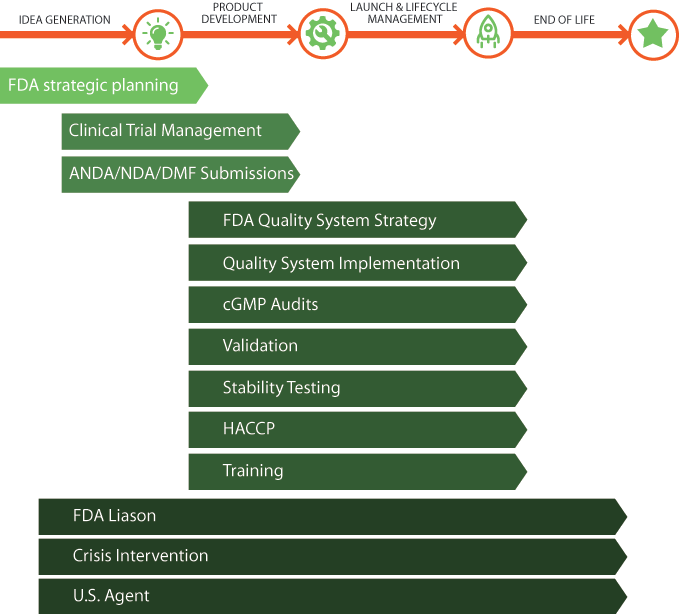
Because FDA inspections can be one of the most trying times in dealing with the FDA, knowing what to expect, interpreting the FDA findings, and providing immediate solutions to prevent regulatory actions is critical to the survival of a company.
mdi Consultants provide ongoing support to let you to work together with our experts throughout the year. With our Liaison Service, when an FDA event occurs or a question arises, we are there to help you. We can deal with:
- FDA inspections.
- Responses to the 483.
- Responses to the W/L