FDA update
RE: FDA Announces the Availability of a Proposed Rule: Revising the National Drug Code Format and Drug
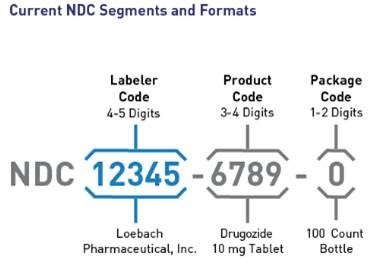
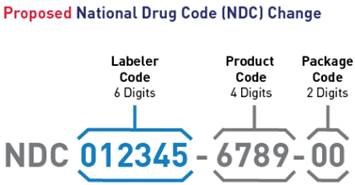
On July 22, 2022, FDA announced the availability of a proposed rule to adopt a single, uniform 12-digit format (a universal 6-4-2 format) for FDA-assigned NDCs. In addition, the FDA proposes to revise the label requirements corresponding to the change in NDC format and, to drug product barcode label requirements. The FDA proposes an effective date five years after the publication of the final rule to allow stakeholders time to develop and implement changes to their systems. A transition period for labeling will last three years after the effective date. FDA encourages manufacturers and distributors to include 12-digit NDCs on their drug labeling as soon as possible after the effective date. FDA intends to mitigate the risk of medication error and confusion during the transition period by maintaining and publishing both the 10-digit and 12-digit NDC formats for specific drugs.
This proposed rule will affect drug products that are required to be listed under section 510 of the FD&C Act and 21 CFR part 207. Once effective, existing 10-digit NDCs will be required to convert to the new uniform 12-digit NDC format, and new NDCs will be assigned in the 12-digit format. However, to reduce the burden on registrants, FDA does not intend to require resubmission of all existing drug listing files. Instead, FDA intends to convert existing NDCs on its own on the effective date by adding leading zeros to the appropriate segments.
Currently NDCs are utilized across the healthcare system. Changes to the NDC would impact human and animal drug manufacturers, insurers/payors, wholesale distributors, drug databanks, pharmacies, hospitals, small clinics and healthcare practitioners, dentist offices, prisons, nursing care facilities, importers, federal agencies using the NDC, state and local governments, and other supply chain stakeholders that use FDA-assigned NDCs. The benefit of the proposed rule, if finalized, would facilitate the adoption of a single NDC format by all stakeholders. This would eliminate the need to convert NDCs from one of FDA’s format to a different standardized format utilized by other sectors of the healthcare industry.
FDA has also proposed to revise the drug barcode label requirements to allow the use of either linear or nonlinear barcodes, so long as the barcode meets the prescribed standards. FDA is considering whether to further revise 21 CFR 201.25(c) to accommodate potential advances in technologies and standards developed by allowing the use of unspecified automatic identification and, data capture (AIDC) formats other than linear or non-linear barcodes in the future without the need to revise the regulation again.
Current National Drug Code (NDC) Segments and Formants
Proposed National Drug Code (NDC) Change
Reference: Proposed Rule on Revising the National Drug Code Format
More info
For more information on the NDC and assistance with assuring other FDA compliance please contact mdi Consultants, Inc. at: info@mdiconsultants.com and ref: NDC