We want to keep everyone updated on the FDA EUA situation and for which devices they have discontinued the EUAs. We knew that the EUA was only temporary under the emergency COVID situation and now the FDA is starting to cut back on the EUAs as they have more supplies available to the marketplace via the 510(k) route.
Here is the most up to date EUA Discontinuance List
As part of the list required pursuant to section 506J(g) of the FD&C Act, the FDA is providing a separate, publicly available, up-to-date list of the devices for which they has been informed by the manufacturer have been permanently discontinued. The FDA will update this list when it receives additional information regarding device discontinuances.
Categories of devices in the EUA discontinuance list are:
- General ICU/Hospital Products
- Infusion Pumps and Related Accessories
- Orthopedic
- Personal Protective Equipment
- Sterilization Products
- Testing Supplies & Equipment
- Ventilation-Related Product – Oxygen Conserver
- Vital Sign Monitoring
| Category | Product Code | Manufacturer Name | Device Trade Name | Reason for Discontinuance | Date Posted (YYYY/MM/DD) |
| Vital Sign Monitoring | FLL (Thermometer, Electronic Clinical) | Philips Medizin Systeme Böblingen GmbH | Tympanic Temperature Module | Discontinuance of the manufacture of the device | 2021/11/23 |
(1)
The table of device types and corresponding product codes identifies devices that FDA believes are critical to the public health during the COVID-19 pandemic under section 506J(a)(1) of the FD&C Act. However, FDA may make additional device type determinations under section 506J(a)(2) of the FD&C Act and update this table with respect to device type recommendations under section 506(J)(a)(1) of the FD&C Act as this public health emergency evolves and if FDA learns new information.
| Device Types | Examples | Product Code |
| Vital Sign Monitoring | Cardiac monitors, Multiparameter Monitors, Respiratory Monitors Thermometers | DQD, PLB, DPS, QDA, MWI, DRG, DRT, MHX, MSX, BZQ, FLL, DQA |
(2)
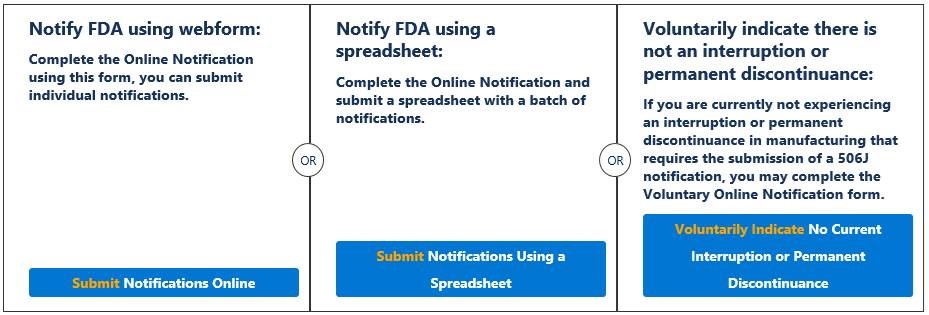
Under Section 506J of the Federal Food, Drug, and Cosmetic Act (FD&C Act), manufacturers of the following devices must notify the FDA of an interruption or permanent discontinuance in manufacturing during or in advance of a public health emergency.
- Devices that are critical to public health during a public health emergency, including those that are life-supporting, life-sustaining, or intended for use in emergency medical care or during surgery.
- Devices for which the FDA determines information on potential meaningful supply disruptions is needed during a public health emergency.
(3)
Cite
(1) Medical Device Shortages During the COVID-19 Public Health Emergency.
(2) Medical Device Types to Help Determine Section 506J Notification Obligations.
(3) Notify the FDA About an Interruption or Permanent Discontinuance in Device Manufacturing (506J Notification).
Remember that you will need a 510(k) to continue to market these medical devices and that you will need to be registered with the FDA and manufacture your devices under the QSR/cGMP (21CFR820).
If you have any questions or require any additional information on the FDA EUA and the discontinuance of these medical devices, please contact mdi at: info@mdiconsultants.com and reference EUA compliance