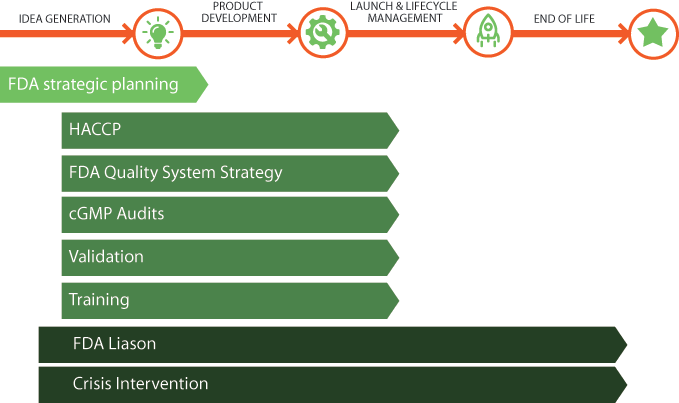
An FDA inspection could be one of the most trying times in dealing with the FDA. Mdi’s Liaison Service knows what to expect, interprets the FDA findings, and provides immediate solutions to prevent regulatory actions, which could be critical to the survival of a company, and provide ongoing support, allowing you to interact with our experts throughout the year.
With mdi Consultants’ Liaison Service, and when a FDA event occurs, or a question arises, we are available to help you.
- mdi’s Liaison Services staff has successfully navigated numerous companies through FDA inspections both domestically and internationally where the consequences of an actionable inspection could prevent product commercialization.
- mdi’s staff of former US FDA field investigators, with over 100 years of combined FDA regulatory experience, provides a professional demeanor to the FDA visit and assists to expedite the inspection in a manner that has been proven over time.
- Our regulatory experience in dealing with the FDA is unsurpassed, with successful removals of injunctions, seizures, and warning letters.
Making these types of decisions requires thought and planning because these decisions will affect the speed and cost of the total implementation, potential savings that could be realized through a quality system implementation and the level of comfort the FDA will have that you are implementing a compliant quality system.